Get Rid of These Two Eye Drops Products Immediately, FDA Warns
Using them could lead to a serious infection.

The Food and Drug Administration is warning consumers to immediately stop using two eye drops products because they’re potentially contaminated with harmful bacteria and fungi. Using them could lead to a serious infection that can be fatal. Read on to find out what two brands you should check your medicine cabinet for right away.
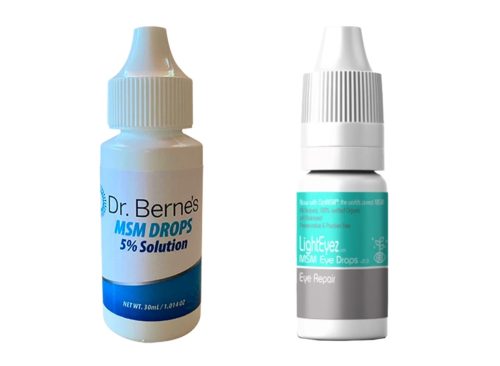
The FDA is advising people not to buy “Dr. Berne’s MSM Drops 5% Solution” and “LightEyez MSM Eye Drops – Eye Repair,” The products may be contaminated with bacteria, fungi, or both, and could cause vision-threatening infections that may be fatal. No problems related to use of the products have been reported so far, the agency said.

If the story seems familiar, you’re right: Earlier this year, the FDA issued multiple warnings against using several eye drop brands linked to an outbreak of drug-resistant bacteria. The outbreak has been connected to dozens of infections and at least four deaths in 18 states.

The FDA advises that consumers discard the products according to the agency’s recommendations. “Using contaminated eye drops could result in minor to serious vision-threatening infection which could possibly progress to a life-threatening infection,” the agency said in a news release, advising anyone with signs or symptoms of an eye infection to “seek medical care immediately.”

The FDA tested the eyedrops and found they were contaminated with microbes and were not sterile, which is required under the Federal Food, Drug, and Cosmetic Act.
According to the FDA, LightEyez’s product was contaminated with Pseudomonas aeruginosa, a bacteria that can cause infections in the blood, lungs or other parts of the body. Health experts think a drug-resistant variant of the bacteria is responsible for the deaths and other health problems tied to eye drops.

Both products also contain methylsulfonylmethane (MSM) as an active ingredient, which is not approved in eye drops sold in the U.S. “These products are unapproved drugs and illegally marketed in the U.S.,” the FDA said in an announcement Tuesday. “There are no legally marketed ophthalmic drugs that contain MSM as an active ingredient.”

Dr. Berne’s eyedrops are distributed by Dr. Berne’s Whole Health Products, and LightEyez Limited is the distributor of Light Eyez products.

Dr. Berne issued a voluntary recall of the Dr. Berne’s MSM Drops 5% Solution, while London-based LightEyez Limited has not responded to the FDA or taken actions to protect consumers, according to the agency.
Pseudomonas aeruginosa is a rod-shaped bacterium less than a millimeter long.”It’s opportunistic, invading any tissue that’s already compromised, and can be lethal: Among the especially vulnerable, the mortality rate can be as high as 50%,” Bloomberg reported last month. “But perhaps the bacterium’s most notable characteristic is how hard it is to kill. The hardiest of pseudomonas are antibiotic-resistant superbugs that rage on no matter what drugs doctors throw at them.”

In February, Global Pharma Healthcare recalled all lots of its EzriCare and Delsam Pharma brands of “Artificial Tears Lubricant Eye Drops,” which it said could be contaminated with bacteria.














